Global Evaluation of the Interstitial Lung Disease (ILD) Diagnostic Pathway in the Post-COVID Era
Objective of study
This study aims to identify characteristics of fibrotic interstitial lung disease (ILD) diagnostic practice and the features, strengths and limitations of distanced virtual multi-disciplinary team (vMDT) meetings, and open discussion on the prevalence of post-COVID fibrosis.
This study aims to:
- Identify the current structure of an vMDT in the post-COVID era
- Identify a standardised approach which can be developed for the digital environment
- Identify limitations and resources needed for vMDTs
- Produce guidance as to how best to optimise the pathway to ILD diagnosis in real-world practice via distance meetings, and on how best to integrate physicians outside their own institutions
- Capture opinion and challenges on post-COVID fibrosis and COVID-associated complications
Rationale
Early diagnosis for ILD and the differentiation between ILDs has been emphasised in order to slow progression, optimise potential therapeutic benefits and increase survival in ILD patients. The multi-disciplinary team (MDT) meeting is the gold standard for diagnosis and management of ILD, increasing diagnostic accuracy and confidence. Regular MDT meeting attendance has been shown to be associated with improved diagnostic reproducibility and allows for knowledge exchange and broadening of expertise.
The COVID-19 pandemic has had major implications and put pressures on national health systems and revolutionised patient management by remote triaging of patient care via distance consultations and telemedicine (TM) (also known as virtual consultation or telehealth). This will have serious implications for diagnosis of ILDs where face to face MDTs are not possible, where the new digital age of healthcare will involve virtual MDTs (vMDTs). It has been recommended that MDTs should include a pulmonologist, radiologist and pathologist to diagnose ILDs, where diagnosis is appropriate. In everyday routine care, specialists are separated by time, geographic location and different schedules. These challenges are further exacerbated in settings where care is provided outside a designated ILD-centre. Through TM, vMDTs may alleviate these problems, and the speed in which specialist opinion can be obtained can be increased.
However, there are global differences in available technology and approaches to TM, where many countries have no regulatory framework to authorise and integrate TM, leading to potential differences in available technology in each country. The post-COVID era in which the world now enters will find digitally enabled care being a mainstay in patient diagnosis and management. COVID has already had a major impact on all areas of ILD patient management, including the diagnostic process, and modifications for initial ILD assessment have had to be made, particularly with provisional diagnosis, as well as patient management post-diagnosis.
The risk of acute exacerbations and progression of ILDs is associated with severe COVID infection, and there is potential for antifibrotic therapy to reduce risk of exacerbation. It has been suggested that there may be substantial consequences for ILD patients following COVID infection, and the risk-benefit ratios of immunosuppressive therapies may be changed.
This study aims to identify key characteristics, priorities, benefits and difficulties of vMDTs associated with ILD diagnosis in the post-COVID era, where face-to-face meetings are not possible, as well as a discussion of potential challenges, complications and caseload associated with post-COVID fibrosis.
Proposed methodology and outputs
This study is a continuation of our previous study on ILD MDTs, available at https://openres.ersjournals.com/content/5/2/00209-2018
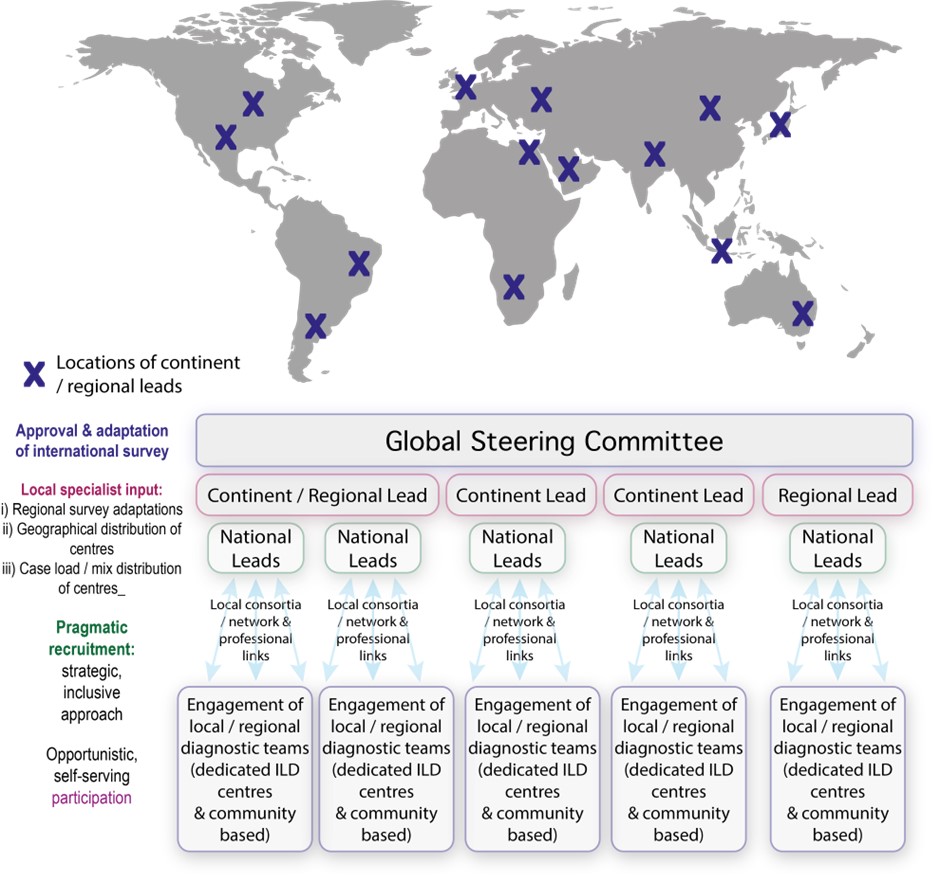
This study will address important questions about current ILD diagnostic approaches around the world in the post-COVID era; and will include dedicated and non-dedicated ILD centres and countries within both mature and expanding economies (featuring a wide range of health systems and health infrastructures) across key global regions. The study will take an inclusive approach via online survey, welcoming responses from all countries and participants involved in the diagnosis of ILD.
Lead collaborators, within prioritised countries and regions, will act as data collection “nodes” – providing local expertise on the geographical distribution of diagnostic centres and the weighting of diagnostic case load across centres. Through local consortia, networks and professional links, these data collection nodes will distribute the survey (and curate responses) within their assigned territory.
Outcomes
The global evaluation of ILD diagnostic agreement will characterise key aspects of the ILD diagnostic pathway and process across the participating centres in the post-COVID era across and between national, regional and international healthcare settings and will result in:
- Descriptive analysis of participating vMDTs:
- Organisation & structure
- Governance
- Information generated in meetings
- Descriptive analysis of vMDT diagnostic practice including:
- Participants’ clinical experience
- Type of centre (e.g., dedicated ILD centre / non-dedicated ILD centre)
- Distribution of ILD diagnoses
- Types of test performed
- Role of available and limited resources e.g., pulmonary function tests, CT scan, autoimmune screen, precipitins, bronchoscopy, genetic testing
- Descriptive analysis of ILD caseload & treatment:
- Patient demographics:
- Number of ILD patients (i) managing and (ii) diagnosing
- Referral pattern of patients (from other- centre vs within- centre vs direct)
- ILD patient management
- Access to licensed antifibrotics
- Patient demographics:
- Descriptive analysis of the following ILD subgroups:
- Idiopathic Pulmonary Fibrosis (IPF)
- Progressive fibrosing ILD non IPF
- Post-COVID ILD
- Identification of the advantages and disadvantages of changing from MDT to vMDT:
- Changes in structure/governance
- Caseload management
- Time to consensus of diagnosis
- Outline of the difficulties and priorities in the evaluation of effect of COVID on ILD patients and associated post-COVID related complications to offer insight into:
- Prognosis evaluation
- Progressive fibrosing ILD post SARS-CoV-2 infection, in patients with previously known ILD
- Frequency and diagnostic approach in patients with post-COVID ILD/fibrosis without previous signs or diagnosis of interstitial lung disease
- Disease behaviour of post-COVID ILD with respect to radiological pattern
- Identification of key issues, requirements and limitations of vMDTs and diagnostic pathways in order to provide guidance about how to optimise the vMDT diagnostic process, e.g.,
- Comparison of caseload - meeting frequency/length
- Clinical expertise/resources - time to diagnosis
Steering Committee
Pilar Rivera Ortega, Global PI, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK
Rayid Abdulqawi, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Gina Amanda, Department of pulmonology and respiratory medicine, Jakarta Islamic Hospital, Cempaka Putih, Indonesia
Katerina Antoniou, Department of Thoracic Medicine, University of Crete, Rethymno, Greece
Azuma Arata, Nippon Medical School, Tokyo, Japan
Ahmed Bayoumy, Suez Canal University Hospitals, Ismailia, Egypt
Milind Baldi, Bombay Hospital, Indore, India
Jürgen Behr, Department of Medicine, University Hospital, Ludwig-Maximilians Universitat München, Munich, Germany
Demosthenes Bouros, Division of Respiratory Diseases, National & Kapodistrian University of Athens, Athens, Greece
Kevin Brown, Department of Medicine, National Jewish Health, Denver, CO, USA
Nazia Chaudhuri, Interim Senior Clinical Lecturer University of Ulster, Northern Ireland, UK
Tamera Corte, Department of Respiratory Medicine, University of Sydney, Sydney, Australia
Vincent Cottin, Department of Respiratory Medicine, Université Lyon 1, Lyon, France
Bruno Crestani, Hôpital Bichat - Claude-Bernard, Paris-Diderot University, Paris, France
Kevin Flaherty, Taubman Center, University of Michigan, Ann Arbor, MI, USA
Ian Glaspole, Central Clinical School, Alfred Hospital and Monash University, Melbourne, Australia
Leticia Kawano-Dourado, Cor Research Institute / University of Sao Paulo, Brazil
Michael Keane, School of Medicine, University College Dublin, Ireland
Martin Kolb, St. Joseph’s Healthcare, McMaster University, Hamilton, Ontario, Canada
Fernando J Martinez, Pulmonary and Critical Care Medicine Division, Cornell University, Ithica, NY, USA
Maria Molina, Institut d'Investigació Biomèdica de Bellvitge, Barcelona, Spain
Ferran Morell, Department of Pulmonology, University Hospital Vall d'Hebron, Barcelona, Spain
Iñigo Ojanguren, Department of Pulmonology, University Hospital Vall d'Hebron, Barcelona, Spain
Laurence Pearmain, School of Medical Sciences, Manchester University NHS Foundation Trust, Manchester, UK
Ganesh Raghu, UW Medical center, University of Washington, Seattle, WA, USA
Paola Rottoli, Department of Medicine, Surgery and Neuroscience, Università degli Studi di Siena, Siena, Italy
Stefan Cristian Stanel, University Hospital Of South Manchester NHS Foundation Trust, Manchester, UK
Gabriela Tabaj, Hospital Cetrangolo, Buenos Aires, Argentina
Carlo Vancheri, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
Brenda Varela, Hospital Alemán, Buenos Aires, Argentina
Bonnie Wang, University of Michigan Health System, Division of Pulmonary and Critical Care Medicine, MI, USA
Athol Wells, Royal Brompton Hospital, Imperial College, London, UK
Graham Lough, REG Scientific Researcher
Status
The survey is now closed and the data is being analysed. Thank you to everyone who participated in the survey.
If you would like further information, please contact Graham (graham@regresearchnetwork.org).
Study registration
ENCePP number: EUPAS47537
Funding
Boehringer Ingelheim